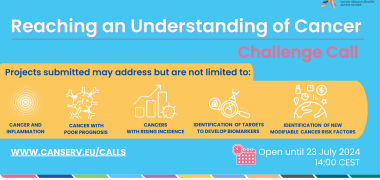
Image

Facilitating European Clinical Research
ECRIN, the European Clinical Research Infrastructure Network, facilitates multinational clinical research, through the provision of advice and services for the set-up and management of investigator or SME led clinical studies in Europe. ECRIN unites national networks of clinical trial units across Europe, through its scientific partners, to fulfil its vision of generating scientific evidence to optimise medical practice.
Image

Image

Image

13
Members & Observer Countries
120
CTUs in our network
361
Million citizens represented
70
Trials in ECRIN portfolio
Supporting clinical research
We provide our 13 Member and Observer countries with diverse trial support services
and contribute to infrastructure development projects with European and international partners.